Shedding Light on the Situation
Monday 12th Oct 2015, 10.00am
Light is more than just light bulbs and sunshine! Researchers at the University of Oxford use different types of light to learn more about all sorts of interesting things. To celebrate the International Year of Light we’ve taken a tiny handful of examples from across the University of Oxford to show just some of the ways light helps us see the world around us.
What did the early universe look like?
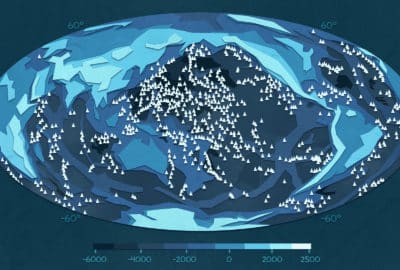
The universe existed in a hot dense space until it suddenly started expanding – what’s known as the big bang. The big bang created a lot of light and we can see the ‘afterglow’ of this as the Cosmic Microwave Background, or CMB for short. As the name might suggest its light in the form of microwaves that is present as background all across the space we can see. It now exists as microwaves because since the big bang, the universe has been expanding, and so the light waves have been expanded (image). This is known as ‘red-shift’ and is similar to why a car goes from a higher pitch to a lower pitch sound as it passes. Using telescopes that can pick up this signal, researchers can build up a picture of what this light looks like. Whilst at first this image looks to be smooth, they’ve found that the picture isn’t completely even; it has lighter and darker patches. By studying this image, researchers can start to understand what the early universe looked like and why stars and galaxies formed in the way they did.
How can we study cosmic phenomena in a lab here on Earth?
Jena Meinecke
Supernova explosions are one of the most powerful events in the known universe—the violent death of a star. They radiate unprecedented energy, outshine entire galaxies, and release elements that make up our physical bodies. In fact, your left hand may have come from a different exploding star than your right hand. Supernovas are also an important birthplace for primordial, “grandparent”, magnetic fields that played a crucial role in the formation of large-scale objects such as our own galaxy. However, the origin of these fields is one of the greatest mysteries facing contemporary astronomers.
How did magnetic fields come into existence?
Scientists believe that magnetic fields did not exist after the Big Bang, but rather were born years later as the universe began to take shape. Cosmic observations have indicated that all matter in the universe is magnetised—from clusters (where galaxies are found) to filaments to voids. The most widely accepted theory is that tiny primordial fields were first generated from asymmetrical shockwaves due to misaligned density and temperature gradients, and then amplified by turbulence.
Creating table top supernovas…
To test this theory, a team at the University of Oxford, led by Jena Meinecke and Gianluca Gregori, is using one of the most powerful lasers in the world to recreate supernovas that fit in the palm of your hand. These shockwaves are similar to supernova remnants such as Cassiopeia A located nearby in the Milky Way. A carbon rod as thin as a strand of hair is ablated (vapourised) inside an ambient gas-filled chamber with six Vulcan laser beams at the Rutherford Appleton Laboratory, reaching millions of degrees Celsius in a billionth of a second. This exploding material expands ballistically outwards, generating a shockwave that has been observed to create its own primordial magnetic fields (Gregori et al. Nature).
Using table top supernovas to piece together the story of the universe…
To amplify these tiny fields, a plastic grid is placed in the path of the laser-produced shockwave to create a turbulent environment. A similar event occurs in the universe as an expanding supernova encounters dense cloudbanks and becomes chaotically tangled. The team’s experiments show that induced turbulence can stretch, twist, and fold magnetic fields like a rubber band, resulting in an overall gain in magnetic field strength (Meinecke et al. Nature Physics). Due to the high impact of these results, the team’s work has been named one of the Top Ten Breakthroughs of 2014 by Physics World. Most importantly, this demonstrates the incredible role lasers now play in piecing together the story of our universe.
Read: Physics World – ‘Lasers ignite ‘supernovae’ in the lab’ – 6th June 2014
How can we ‘see’ what’s going on during chemical reactions?
Dr Chris Rennick and Dr Brianna Heazlewood
Using continuous-wave diode lasers in the UV (at 397 nm) and IR (at 866 nm), we can literally cool down calcium ions held within an ion trap. Laser cooling works by exploiting the wave-matter duality of light (sometimes light acts as a particle, and sometimes a wave); if an ion is travelling towards a laser beam and absorbs a photon, the velocity of the ion will decrease as the photon has momentum and momentum (p=mv) must be conserved in a collision (imagine trying to slow down a training by throwing ping-pong balls at it). When the ion relaxes back to the ground state, it emits photons in all directions, resulting in a net loss of momentum in cases where the velocities of the ion and the absorbed photon are opposed. To ensure that the trapped ions preferentially absorb photons travelling towards them – as opposed to photons travelling in the same direction, which would increase the velocity of the ions – we employ the Doppler effect. The cooling laser is slightly detuned below the resonant absorption energy, such that photons travelling towards the ion are Doppler shifted to match the resonant frequency of the transition. On the order of 107 laser cooling cycles can occur per second, and so the velocity of the ions can be reduced significantly in a very short period of time. As the laser-cooled calcium ions are continuously fluorescing, we can observe the emitted photos using a microscope lens and a camera. This allows us to literally watch reactions occur on a microscopic scale, in real time. In our experiments we can precisely control the properties of the reactants and can detect any product ions formed. This enables us to examine how ion-molecule reactions happen under cold conditions, such as you would find in the outer atmosphere and in space. We know that the rates of ion-molecule reactions increase as the temperature decreases – the opposite to what occurs in most reaction systems. As a result, ion-molecule reactions become increasingly important in regions of low temperature, such as the outer atmosphere and the interstellar medium.
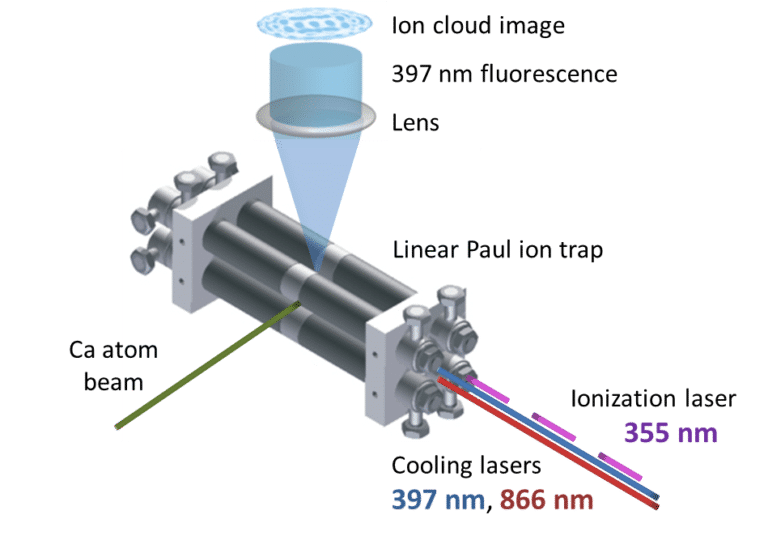
Fig. 1. Schematic showing the ion trap, the lasers and the fluorescing calcium ions. Figure reproduced from: B. R. Heazlewood & T. P. Softley, Annu. Rev. Phys. Chem. 66, 475 (2015).
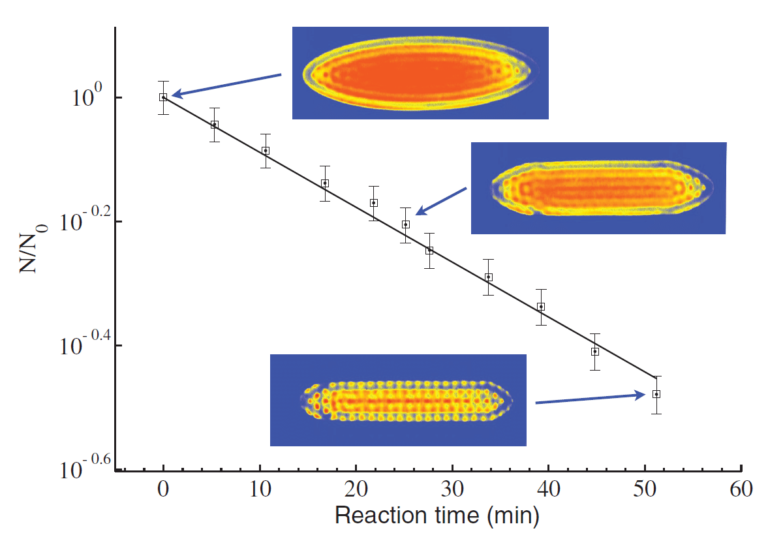
Fig. 2. The reaction between Coulomb-crystallised calcium ions and neutral reactant molecules (in this instance, CH3F) is shown as a function of time. Each of the dots in the experimental images represents the lattice position of a calcium ion. As the calcium ions react, two changes can be observed in the crystal images: the number of fluorescing ions decreases and the crystal appears flatter. This is due to the formation of CaF+ product ions, which sit outside the remaining Ca+ ions but cannot be seen by the camera as they are not laser cooled and thus do not fluoresce. Figure reproduced from: S. Willitsch, M. T. Bell, A. D. Gingell & T. P. Softley, Phys. Chem. Chem. Phys. 10, 7200 (2008).
Watch a chemical reaction taking place in real-time here!
At the beginning of the movie, you see a Coulomb crystal formed entirely of fluorescing Ca+ ions. Xe+ ions are then incorporated. While Xe+ ions are not laser cooled and therefore do not fluoresce, they induce changes in the crystal: the crystal gets hotter as the laser-cooled Ca+ ions “sympathetically” cool the Xe+ ions, resulting in a diffuse cloud-like structure rather than the previously sharply resolved crystal lattice. When the crystal cools down again (after sufficient laser-cooling cycles have removed the excess energy of the Xe+ ions through collisions with Ca+ ions), the fluorescing Ca+ framework appears flatter and elongated. This is because Xe+ ions are heavier than Ca+ and thus sit outside the Ca+ ions. After a short period of time, neutral deuterated ammonia (ND3) molecules are admitted to the reaction chamber. These ND3 molecules undergo a charge exchange reaction with Xe+, forming ND3+ ions. Again, while we cannot see the ND3+ ions (they are not laser cooled and thus do not fluoresce), we can observe changes in the crystal due to their presence. A dark core begins to form, as ND3+ is lighter than Ca+, and this grows over time as the charge exchange reaction proceeds. Note that Ca+ ions are spectators in the reaction; the number of Ca+ ions is unchanged, as they serve to cool the other ionic species through elastic collisions, and they also allow us to visually follow the reaction progress.
How can we test potential new drugs?
Dr Clarence Yapp
The cell membrane of cells is very effective at keeping foreign molecules such as drugs and toxins out of the cell. Unfortunately, this can create complications in early drug development programmes, where drugs are effective but suffer from cell impermeability. For example, we may want to test whether a new drug has any therapeutic effect on cancer cells, but since the drug cannot enter the cell, we may never know of its true potential for later redesign. Using a femtosecond pulsed laser in the infrared region, we have temporarily permeabilised the membrane of cells to introduce foreign ‘cargo’ to address this problem. The cargo can be in the form of drugs or proteins. This technique is called optoporation and, unlike other methods of permeabilisation, it is less harmful to cells and can be visualised in real-time under a microscope.
How do we look at fossils trapped in rocks?
Dr Matt Friedman
X-ray computed tomography, more widely recognised as CT scanning, is a powerful technique that allows scientists to measure the 3D structures of fossil organisms that are still encased in rock – without having to dismantle or destroy the specimen. The specimen is simply scanned at several angles using X-rays that can pass through the rock and pick up the structures that lie beneath. This produces images that are essentially ‘slices’ through the object, which are then peiced together by a computer, producing the 3D image.
In recent work the brain cases of a set of early fish have been investigated, discovering new features that give clues about the evolution of eyes and other senses early vertebrates.

Fig. 3. The result of a 3D scan of the inside a fossilised primitive fish’s (Kentuckia deani MCZ 8361) brain case. This image is known as an ‘endocast’.
Some other great visualisations can be found here.
Further information:
Shedding Light on the Situation Teaching Resources:
Find out more:
International Year of Light in the UK
‘Towards Absolute Zero’ animation
Dr Brianna Heazlewood presents how the Stark Decelerator works
Make your own spectrometer to examine the composition of light