Chinenye Akpulu
Bacteria Scientist
My name in full is Chinenyenwa, an Igbo name that means “God gives a child”. I work on microorganisms in the body and how sometimes these can become resistant to antibiotics.
My story
I was born and raised in the South-Eastern part of Nigeria in Africa. My dad was a civil engineer, and my mum was a nurse until they both retired a few years ago. Growing up, my parents made it clear that education is important to be successful in life. My dad would always tell us the story of his journey to Germany where he got his degree in civil engineering without any financial aid from his parents, and he was of the opinion that we can be whatever we want to be in life if we put our minds to it.
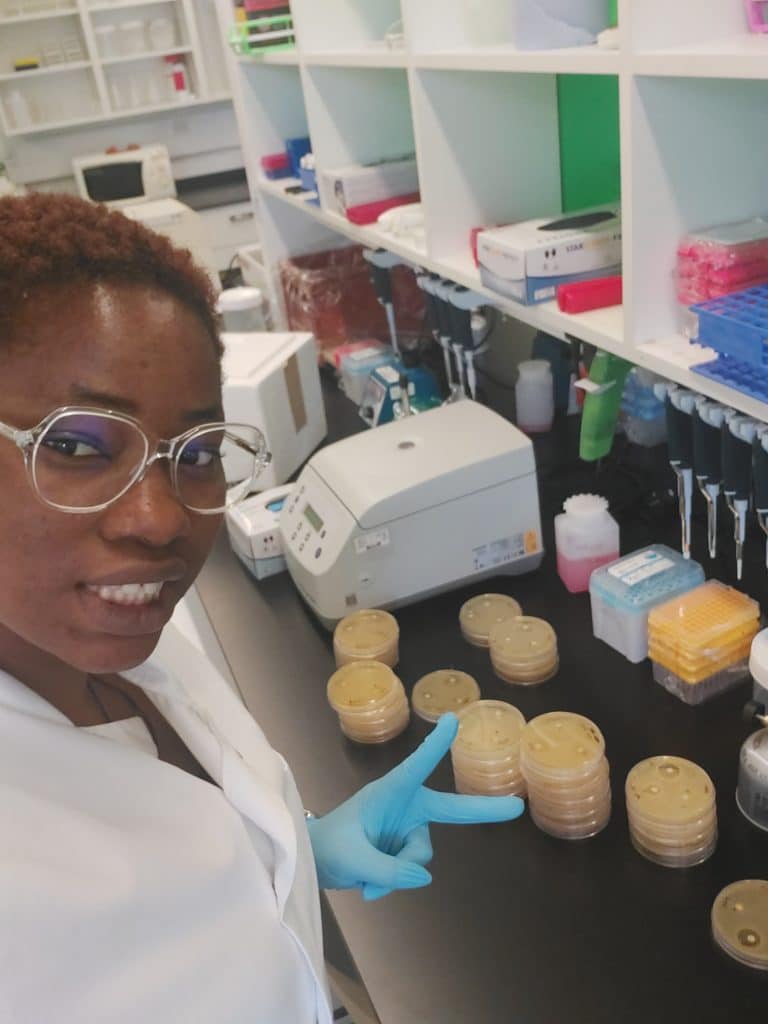
Chy in the lab with bacteria samples
There are lots of different reasons why I have developed an interest in research, specifically research that helps people. I have always been curious and want to find logical explanations as why things are made or formed. As an empath, I tend to notice other people’s emotions, which may explain why I am drawn to research that directly benefits human beings.
My educational journey started in Nigeria. I had my nursery, primary, and secondary education, and did my bachelor’s degree in Nigeria, but went to do my Masters at the University of Glasgow. Traveling to Glasgow was an interesting phase of my life as this was the very first time I left the shores of Nigeria. I had my fair share of culture shock and challenges in Glasgow. I juggled studying for a degree with adapting to a new culture, learning to understand the accents and to be understood, getting involved in a new way of life, and homesickness. The challenges almost stopped me from continuing in my academic pursuit as, after this experience, I promised myself that I was not going to do a Ph.D., and went back to Nigeria to work for a few years. Fortunately, a few events and personal traits inspired me to go back to research and do a Ph.D.

Chy in front of a microbiology lab in AKTH (Aminu Kano Teaching Hospital) Kano, Nigeria. This is one of the sites for the IOI-AMR project
My journey to becoming a researcher started back home in Nigeria in 2016 when I worked as a research assistant with a project group called (BARNARDS). I was inspired by how the project was designed to make use of research findings for the benefit of local communities. The study specifically looked at newborn babies who develop bloodstream infections called ‘Sepsis’, investigating why some already had resistant bacteria even though they’ve never had antibiotics. Resistant bacteria, or ‘antimicrobial resistance’ happens sometimes because of the misuse of antibiotics, especially in countries where antibiotics can be bought without a doctor’s prescription. However, in this study, we found that some newborn babies who had never received antibiotics still have the resistant bacteria and we could not understand why or how they got it. Our theory was that maybe the mothers passed it to the babies when they were born, or they picked it up from the hospital environment or from their caregivers.
Through this job, my interest in research grew, and so did my confidence in knowing that I could make a difference and solve real-world problems. This, along with my curiosity and empathy, helped me decide to join the Walsh Lab within the Ineos Oxford Institute at the University of Oxford.
Asides from my work as a researcher, I love arts and the creativity of bringing arts of any kind to life, especially decoration. From cake decoration (see pictures of some of the cakes I have baked with my sister), to house decoration or fashion. I am also a handy person and always try to fix broken things before I seek the help of a professional. I also love hiking and being in nature.


About my research
My DPhil project looks at how the collection of microorganisms in our bodies develops, and how some of those bacteria may become resistant to drugs. I want to understand what it is that shapes this often neglected ‘organ’ of the human body.
So, let’s start with the basics. The microbiome is the community of microorganisms (such as fungi, bacteria, and viruses) that exist in a particular environment while resistomes are the genes that these microorganisms have which allow them to resist the actions of antibiotics. In humans, the terms are often used to describe the microorganisms and their resistant genes that live in or on a particular part of the body, such as the skin or gastrointestinal tract (the gut). There are many microbes living all over our bodies. They are all up in our nose, mouth, on our skin but they love our stomach the most. So, when we are talking about the gut microbiome, we are referring to all those tiny microbes in our gut and their genes combined.
Picture the gut microbiome as the jungle; instead of trees, plants, and animals, we have microbes which are fungi, bacteria, and viruses. And just like the jungle, different microbes have different jobs, and the more diverse the microbes, the healthier our gut is. Our human body is said to be made up of about 100 trillion cells but carries about ten times as many microorganisms in the gut alone. So technically, we can say that we have 10 times more microbial cells in our bodies than human cells, which is why some people say it is an extra body part.

Chy and Nigerian colleagues in front of a BARNARDS mobile lab in MMSH(Murtala Muhammed Specialist Hospital) Kano, Nigeria. Another site in Kano for IOI-AMR project.
Okay now that we know what the gut microbiome is, why is it important? And why do we care?
Just like how trees are needed for oxygen and insects are needed to keep the soil healthy in the jungle, we need many different types of microbes to carry out different jobs in our bodies to keep us healthy. But there are good (non-pathogenic) and bad microbes (pathogenic). In a healthy person, the microbes live together with no problems but when there are more bad microbes than good microbes (dysbiosis), that’s when we don’t feel too good.
The gut influences our weights, moods, and appetites. It also helps fight off infections, boosts immune systems, and improves digestion. Maybe that’s where we got the saying, “trust your gut”!
Each of us has our unique gut which is shaped in our first two-to-three years of life. Environment, diet, and the drugs we take are known to play a part in shaping our gut microbiomes. But the most instant effect is made by the antibiotics we take when we get sick.
Researchers study antibiotic resistance by growing a disease-causing microorganism – or pathogen – in a Petri dish and then feeding the pathogens with antibiotics. If the pathogens can grow in the presence of antibiotics, they are said to be “resistant” (this is how doctors choose antibiotics for treating patients with infections). However, the tricky part is that our gut contains thousands of different microbes and the pathogen making us sick is just one of these thousands. So, when we senselessly take antibiotics, we kill both the good and bad microbes.
Picture this as slashing and burning down a whole jungle. And when this happens, the jungle which is our gut may not be able to grow back to its diverse healthy state. This instability in the gut microbiome has been linked to many diseases like allergies, asthma, autism, irritable bowel syndrome, diabetes, and obesity. And they can also act as a reservoir for antimicrobial resistance genes where the resistant genes are shared between the bad and the good microbes. This aspect is the basis for my project.

Chy with her Supervisors Prof Tim Walsh and Dr. Sands, and a Postdoc Dr. Portal on a scoping visit to the Neonatal Unit at National Hospital Abuja. Gearing up for the second phase of the BARNARDS project
So, we know that the gut is important, but this field of research is still in its early stages. We don’t know yet what a healthy gut microbiome looks exactly like, we don’t know what types and amounts of microbes are beneficial from person to person. We know that some microbes in the gut carry the resistant genes that make them resistant to antibiotics when they cause infections, but we do not know how newborn babies get these microbes or how the microbes acquire these genes. This is what my research is trying to understand.
Antimicrobial resistance is a global threat and despite all the efforts to solve the problem, it remains a huge public health threat and its highest burden is in developing countries. If too many disease-causing microorganisms are resistant to the drugs that should treat them, it would be like going back to a time when an infection from something as simple as a cut could have awful consequences. For my project, I am looking at how these microbes in a baby’s gut develop from when a baby is born until when they are two years old at which point the composition of the microbes is said to resemble that of an adult. The findings from my research will add to our current knowledge of antimicrobial resistance and bloodstream infections and it may give us ideas on what strategies we can use to prevent and treat infections in babies.


