Oxford spinout trials revolutionary bioelectronic implant to treat incontinence
Tuesday 31st Jan 2023, 10.14pm
Amber Therapeutics, a company spun out from the University of Oxford in 2021, applies a research system which uses ‘closed-loop neuromodulation’. This is a type of therapy which involves implanting a device which can sense, interpret, adapt and respond to individual patient signals, in an attempt to restore normal bladder function.
Investigators are exploring how to directly regulate the urge to empty the bladder (‘urge incontinence’) and also increase resistance to urine leakage caused by activities such as coughing or lifting (mixed urinary incontinence’). The device is inserted in participants’ pelvic region using a minimally invasive surgical procedure that accesses and targets the nerve that directly controls continence.
Modern bioelectronic systems have the unique capability to measure physiological signals and adjust stimulation in real-time. In partnership with clinicians, we can create novel adaptive, reflex-like, algorithms for exploring new therapies. The flexibility of these systems allows us to use software updates to configure to different disease states. This study builds on the prior trial in Multiple System Atrophy and paves the way for emerging therapies in generalized epilepsy and chronic pain.
Professor Tim Denison
To date, five participants have been safely implanted with the system, known as Picostim-DyNeuMo, in which an adaptive algorithm is activated and runs continuously in an at-home setting. The remaining participants will be enrolled during the first half of 2023.
Early indications confirming the feasibility of the surgical procedure and therapy are very promising for future treatment of one of the most common medical problems in humans, affecting 8.5 per cent of the global population.
The Picostim-DyNeuMo research system was developed in a collaboration between Bioinduction Ltd, a Bristol-based bioelectronics technology company, and Professor Tim Denison, RAEng Chair in Emerging Technology at the Department of Engineering Science and Nuffield Department of Clinical Neurosciences.
The implanted research tool was first implemented last year for the treatment of Parkinson’s-like multiple system atrophy. It has the potential to treat a range of conditions including epilepsy and chronic pain, and several UK universities are now collaborating on applying the tool for experimental medicine.
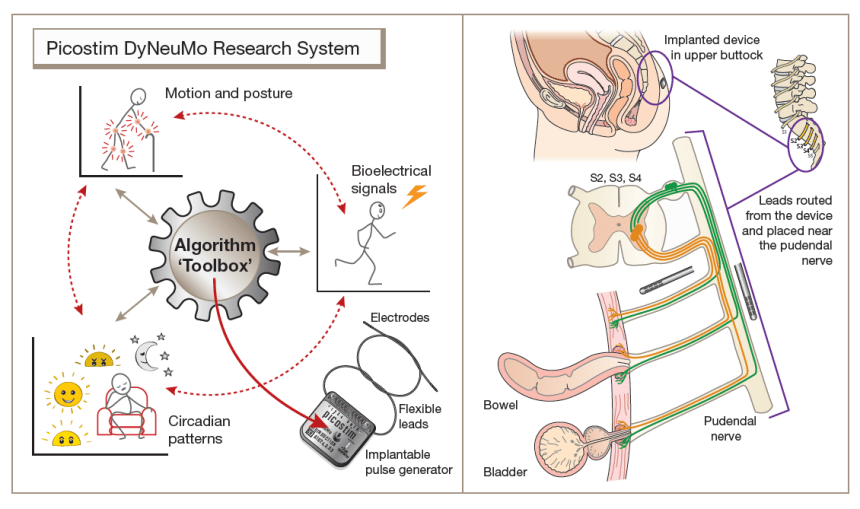 Diagram of the Picostim DyNeuMo research system toolbox
Diagram of the Picostim DyNeuMo research system toolbox Amber Therapeutics started its ground-breaking first-in-human study in late 2022. Professor Denison co-founded Amber Therapeutics with surgeons Dr Charles Knowles and Dr Stefan de Wachter, and entrepreneur-in-residence Aidan Crawley, to accelerate transformational therapy innovation for incontinence.
Oxford Science Enterprises, 8VC and a UKRI Biomedical Catalyst grant provided seed funding for the development work. The trial will evaluate the safety and pilot efficacy of the therapy in 15 women. It is being conducted at the University Hospital Antwerp (Belgium).
Stefan De Wachter, Professor of Urology at Antwerp University and leading investigator for the study, said: ‘Most of the current available implanted therapies for incontinence are static (tapes, slings) or can only influence the bladder indirectly (such as sacral or tibial stimulation). In this trial, we stimulate the pudendal nerve, the natural pathway of continence control, and can reinforce the existing physiologic reflexes when they are needed. With our adaptive therapy, we finally have the potential to control both forms of continence: relaxing the bladder to treat urge and closing the sphincter to treat stress incontinence.’
The study is expected to conclude towards the end of 2023, with learnings used to improve and optimise the therapy in preparation for a large-scale trial.
Read more about the study on the Nuffield Department of Clinical Neurosciences website.

