Bacterial armour plating has implications for antibiotics
Thursday 3rd Nov 2022, 11.48am
A new study published in the journal Science Advances sheds light on how Gram-negative bacteria like E. coli construct their outer membrane to resemble body armour, which has far-reaching implications for the development of antibiotics.
Professor Colin Kleanthous in the Department of Biochemistry at the University of Oxford led the interdisciplinary study, with contributions from colleagues in Oxford and University College London. They undertook a microscopic examination of the outer membrane of E. coli to understand the molecular basis for the protection it affords against many classes of antibiotics. E. coli causes infections such as pneumonia, UTIs and sepsis that are notoriously difficult to treat due to multidrug resistance.
The outer membrane is composed of two types of lipids that stack on top of each other, an unusual arrangement which, it was thought, is solely responsible for making the membrane resistant to antibiotics. As well as lipids, the outer membrane contains numerous proteins which the bacterium relies on to acquire nutrients and excrete waste products. Textbooks classically show these proteins dotted randomly in the membrane, contributing little to its stability or structure.
The discovery of Professor Kleanthous and colleagues came from them asking a simple question: do protein interactions play any role in the structural integrity of the outer membrane?
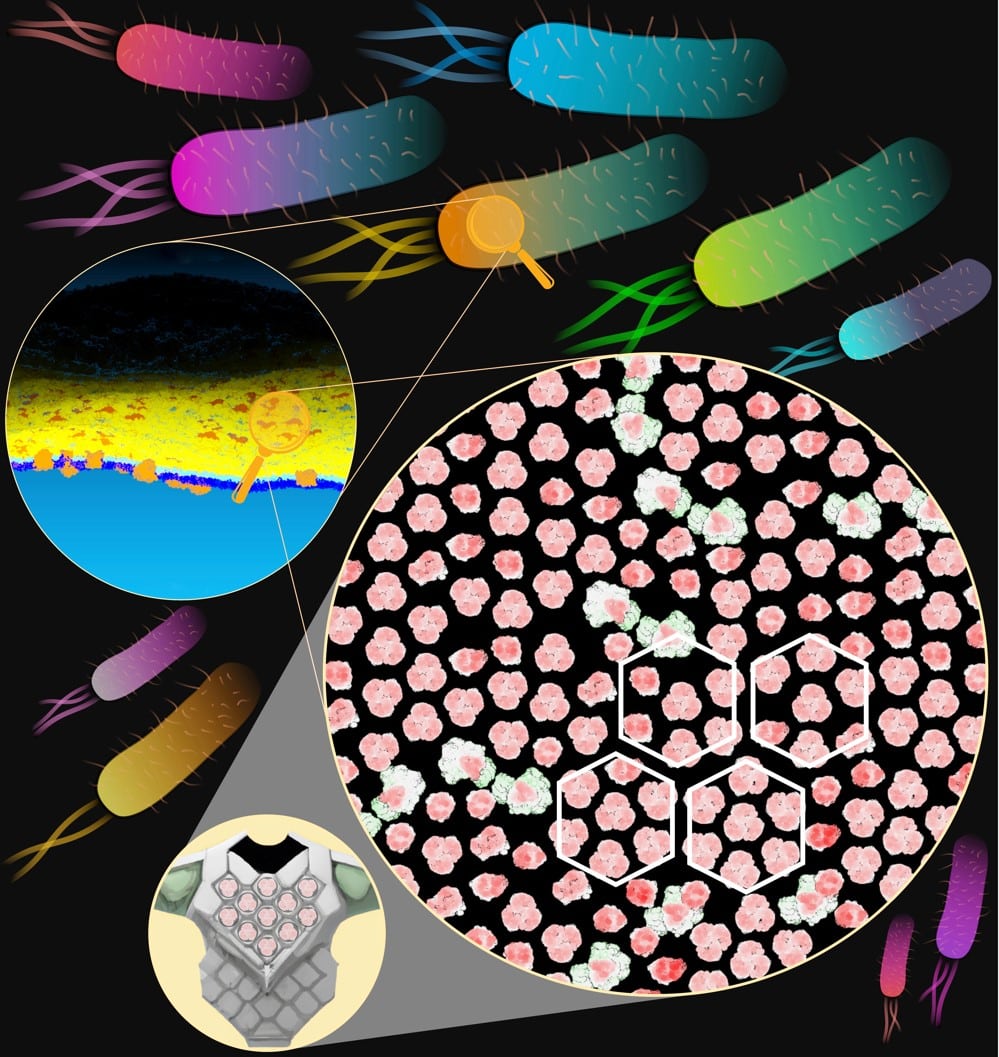
Image: Magnified view of the E. coli outer membrane showing hexagonal clustering of proteins (red/green), alongside body armour for comparison. The black background represents lipids that are shared between neighbouring proteins. Figure kindly provided by Dheeraj Prakaash and Syma Khalid (Department of Biochemistry, University of Oxford).
Although technically challenging to investigate in bacteria, they succeeded in answering the question using state-of-the-art experimental approaches coupled with computer simulations. By tagging the outer face of proteins within the outer membrane with photoreactive chemicals, they found that not only was each protein surrounded by a ring of stacked lipids but that these lipids were shared with neighbouring membrane proteins.
Even more surprising was the finding that the resulting network of promiscuous protein-lipid-protein complexes spans the entire bacterial surface and embedded within it hexagonal lattices reminiscent of those used to strengthen protective body armour.
‘This work completely changes our understanding of the outer membrane, its physical characteristics and how it is built,’ Professor Kleanthous explains. ‘Every protein appears connected to every other protein in the membrane by a network of lipids, creating cellular armour plating that researchers will need to take account of in future antibiotic design.’
The full paper, ‘Lipids Mediate Supramolecular Outer Membrane Protein Assembly in Bacteria‘, is published in the journal Science Advances.
The work was supported by the European Research Council, the Wellcome Trust, the Medical Research Council and the Engineering and Physical Sciences Research Council.

